Fast. Accurate. Quantitative.
- Scintillation Crystal and Silicon Photomultiplier
- Delivered right to the patient
- Machine learning meets precision medicine
Sometimes a simple idea is the best idea
The Lara® System
- Patient Safety/Dosimetry and Quality in Radiopharmaceutical Administrations
- Immunotherapy assessment*
- Palpable tumor assessment*
- Parkinson’s Disease evaluation*
- Rheumatoid Arthritis quantification*
*These applications have not been cleared or approved by the FDA and are limited by United States law to Investigational Use
The first commercial application for the Lara® System: Patient Safety
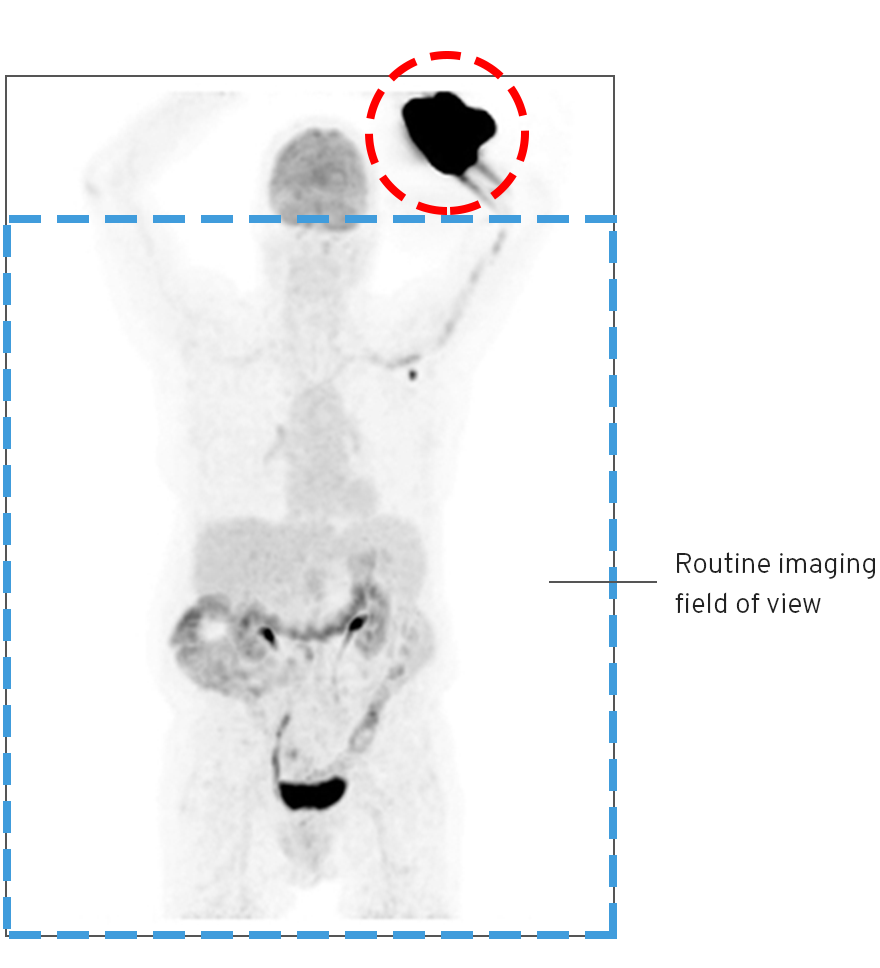
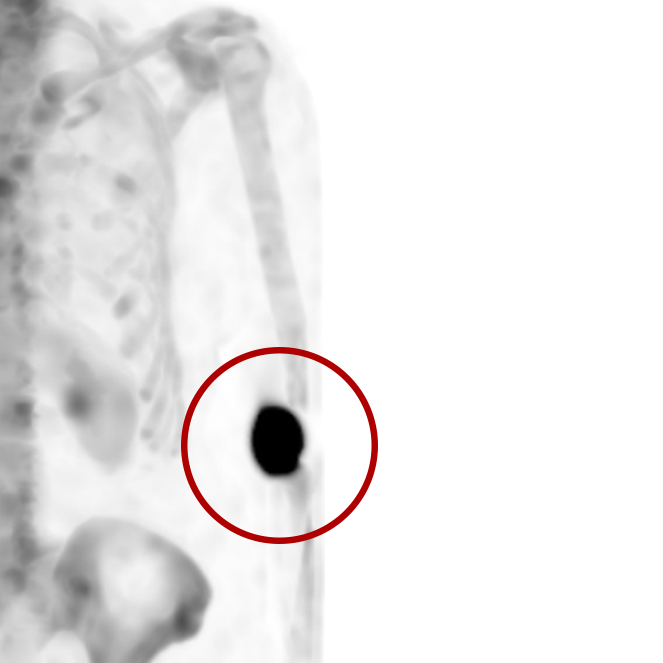
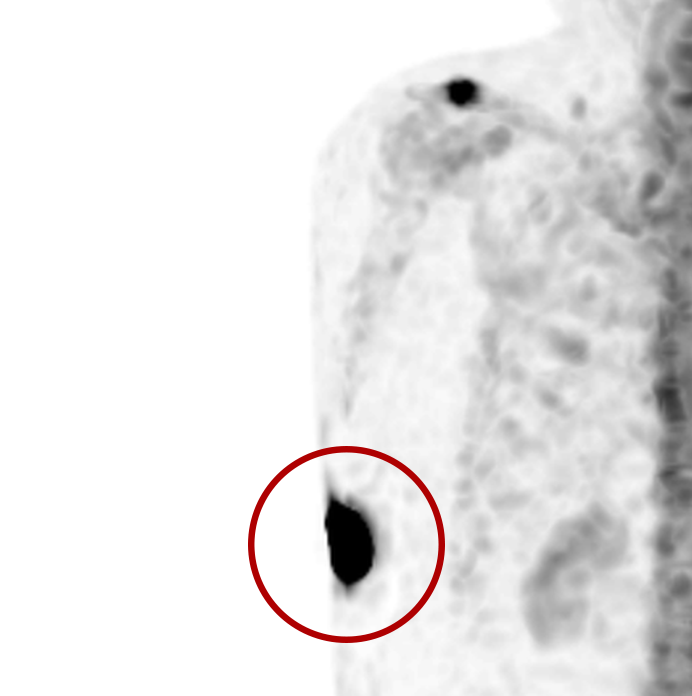
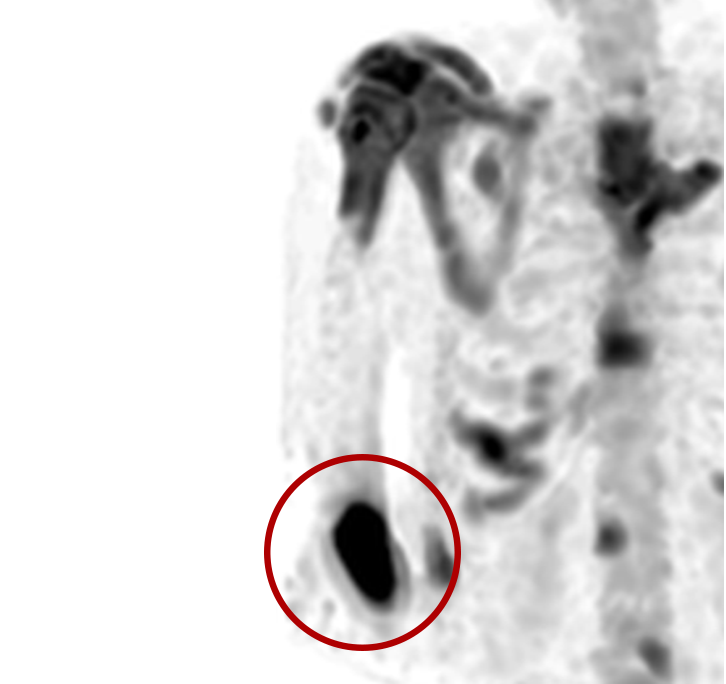
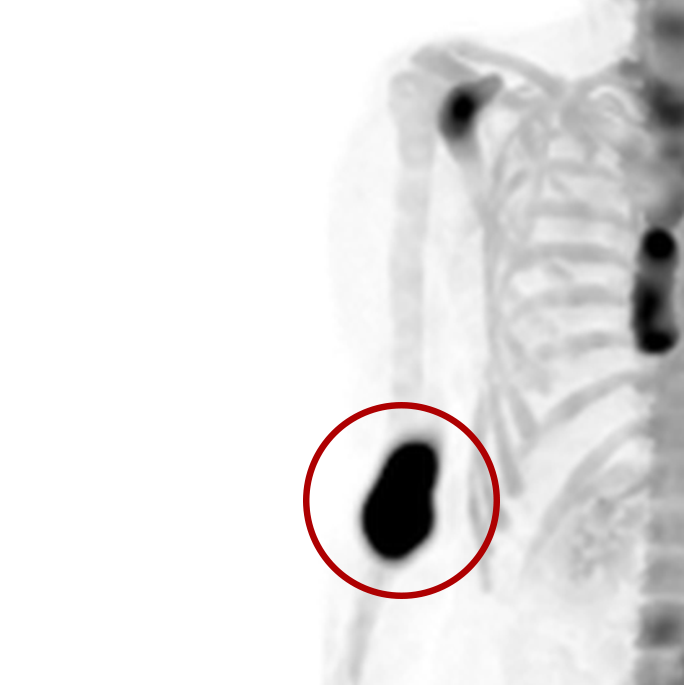
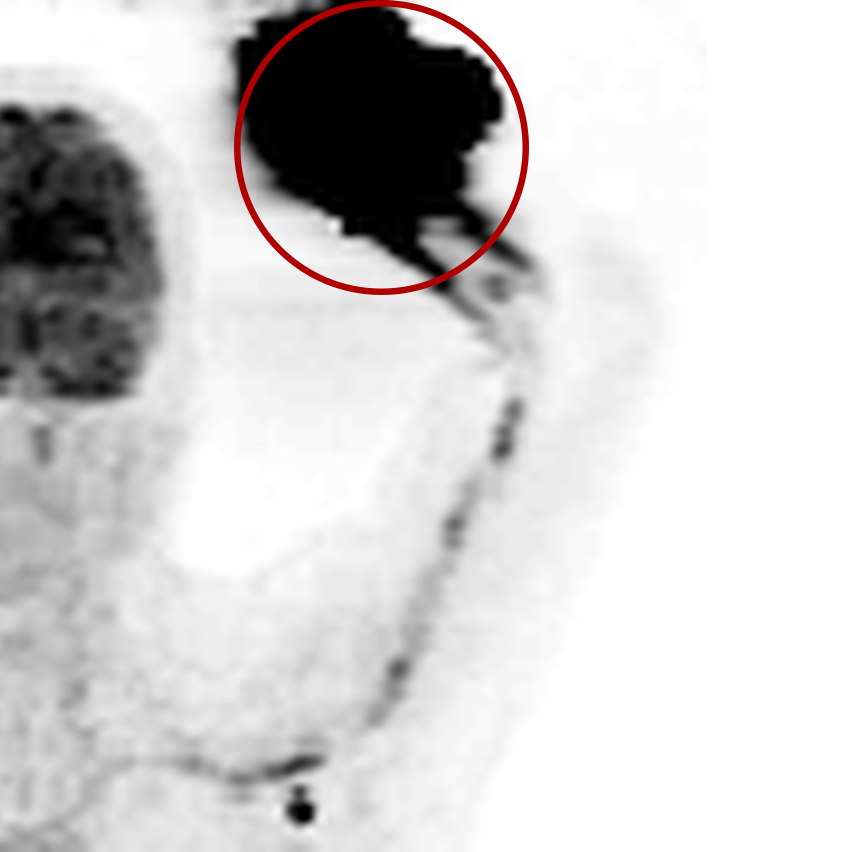
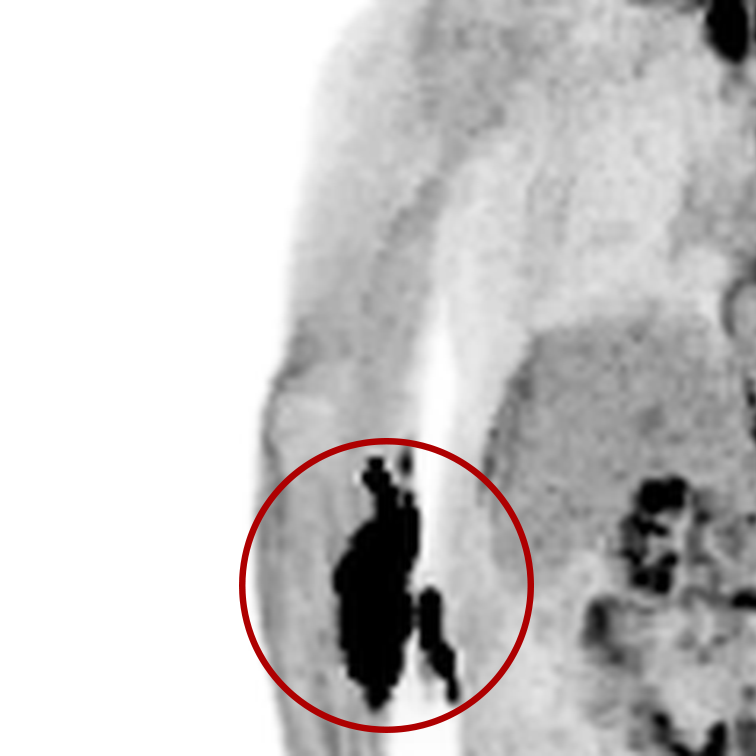
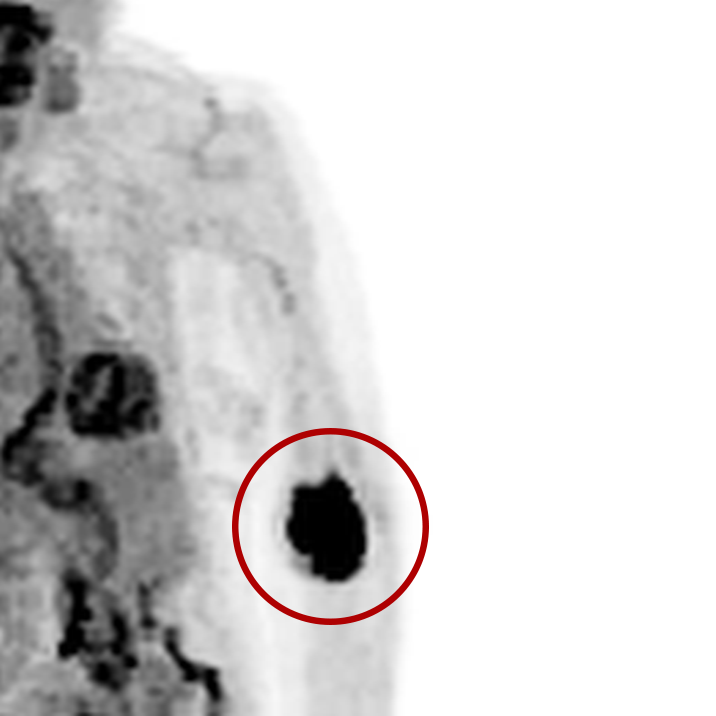
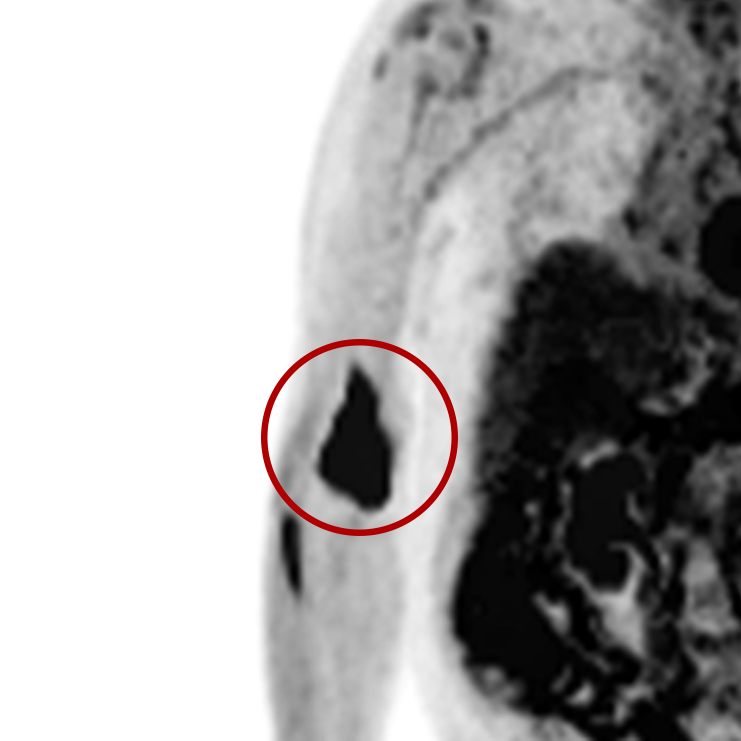
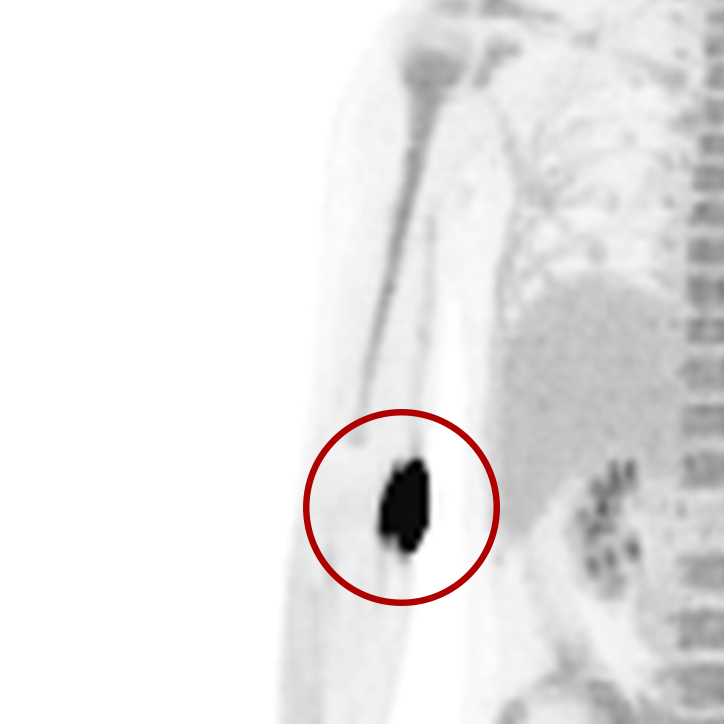
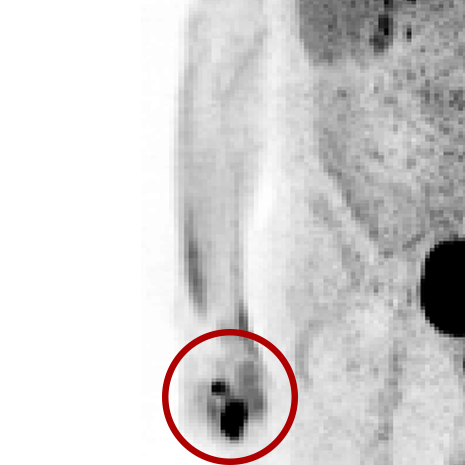
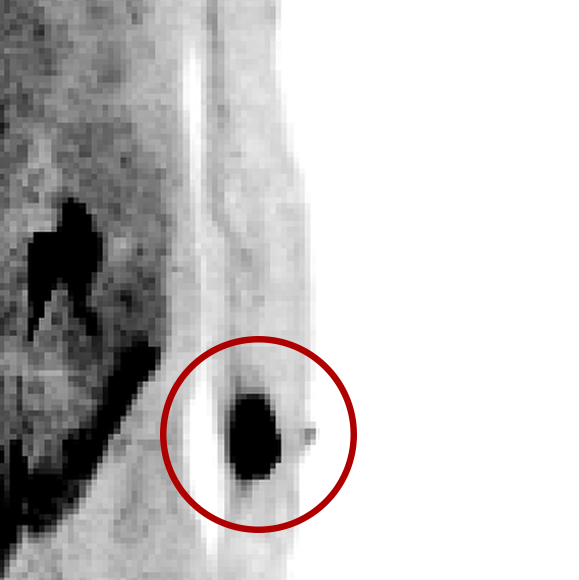
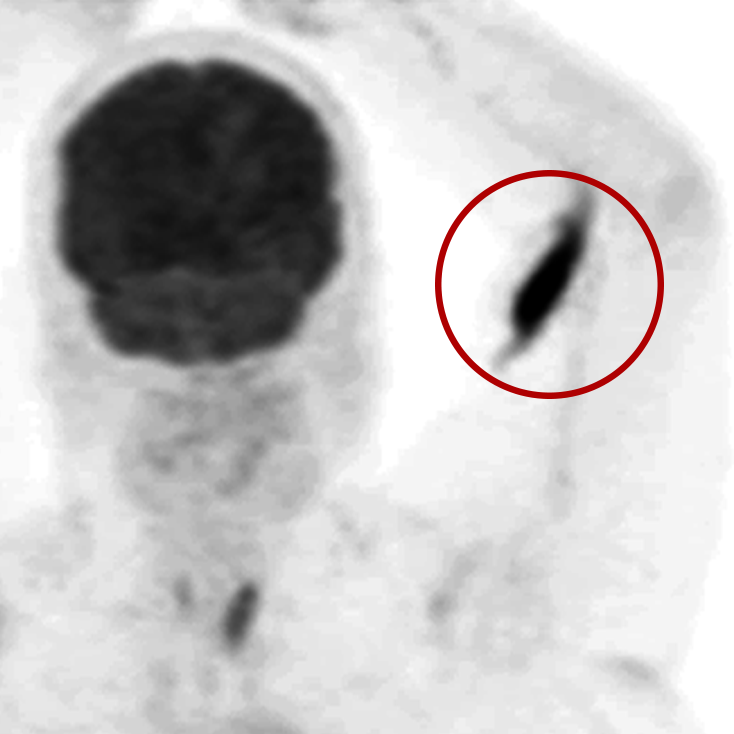
An infiltration is the inadvertent administration of a pharmaceutical into the tissue instead of the vein, as intended. Although an extravasation is typically defined as an infiltration of a vesicant, an infiltration of a radiopharmaceutical can be considered an extravasation due to the effects of ionizing radiation on patient tissue. Lara provides quality control and quality assurance for nuclear medicine administrations and when extravasations occur Lara also provides information that is critical to help calculate accurate patient-specific dosimetry. So, patients know when they have received unintentional radiation dose to their tissue and physicians know when images are compromised.
- Published radiopharmaceutical extravasation rate: 15.5% (8 studies, 12 centers, 3354 patients)**
- Extravasations reduce diagnostic sensitivity
- Extravasations may result in high unintentional dose to tissue
Annually:
including ~30M radiopharmaceutical administrations
***Company estimates based on 22,000+ monitored administrations
Contact us
Interested in learning more about The Lara® System?
Contact us for a conversation about monitoring and improving the quality of your radiopharmaceuticals administrations.
**
- Osman, M.M., et al., FDG dose extravasations in PET/CT: frequency and impact on SUV measurements. Front Oncol, 2011. 1: p. 41.
- Hall, N., et al., Impact of FDG extravasation on SUV measurements in clinical PET/CT. Should we routinely scan the injection site? J Nucl Med, 2006. 47(suppl 1): p. 115P.
- Bains, A., et al., Contamination in 18F-FDG PET/CT: an initial experience. J Nucl Med, 2009. 50 (supplement 2): p. 2222
- Krumrey, S., et al., FDG manual injection verses infusion system: a comparison of dose precision and extravasation. J Nucl Med, 2009. 50(supplement 2): p. 2031.
- Silva-Rodriguez, J., et al., Correction for FDG PET dose extravasations: Monte Carlo validation and quantitative evaluation of patient studies. Med Phys, 2014. 41(5): p. 052502.
- Muzaffar, R., et al., Novel method to detect and characterize (18)F-FDG infiltration at the injection site: a single-institution experience. J Nucl Med Technol, 2017. 45(4): p. 267-271.
- McIntosh, C. and J. Abele, Frequency of Interstitial Radiotracer Injection for Patients Undergoing Bone Scan, in The Canadian Association of Radiologists. 2016: Montreal, Quebec.