The Lucerno Solution for Extravasations
The Lucerno Lara System is currently listed with the FDA to dynamically measure the presence of radiopharmaceutical in an organ or body region during the uptake period as part of nuclear medicine procedures. The system is indicated for use with nuclear medicine patients.
The system is indicated for use as a quality control tool to help assess whether a radiopharmaceutical remains near the administration site rather than circulating in the vascular system. The system is also indicated to help evaluate the biological clearance of a radiopharmaceutical. Additionally, the system is indicated for use as a quality assurance tool to monitor, evaluate and help improve the radiopharmaceutical administration process.
Lara is commercially available.
How Lara works
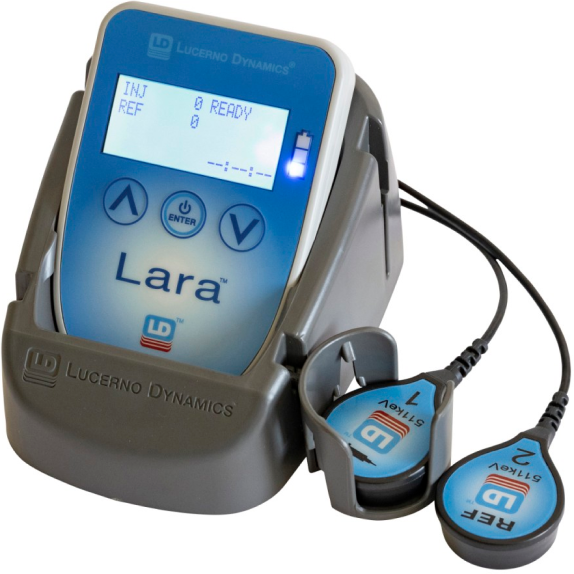
Lara uses sensors on the patient’s skin to detect the radiation emitted from the areas of interest beneath the sensors.
Prior to radiopharmaceutical administration, these sensors are placed on the patient’s arms, using atraumatic adhesive pads. One sensor captures activity from the radiotracer at the administration site and the second sensor from the other arm, which acts as a control. Lara provides a real-time display of administration quality and after a portion of the uptake period, the sensors can be removed and recorded data uploaded to Ellexa® Explorer, Lucerno’s PC application. Within seconds, a time-activity curve is displayed.
The Lara System is agnostic to imaging FOV, technologist experience, vein quality, or vascular access method. Lara is easy to use, requires no additional radiopharmaceutical, and adds only twenty seconds to the patient experience and one to two minutes to the technologist’s process.
Lara provides three distinct benefits: quality control, dosimetry, and quality assurance.
